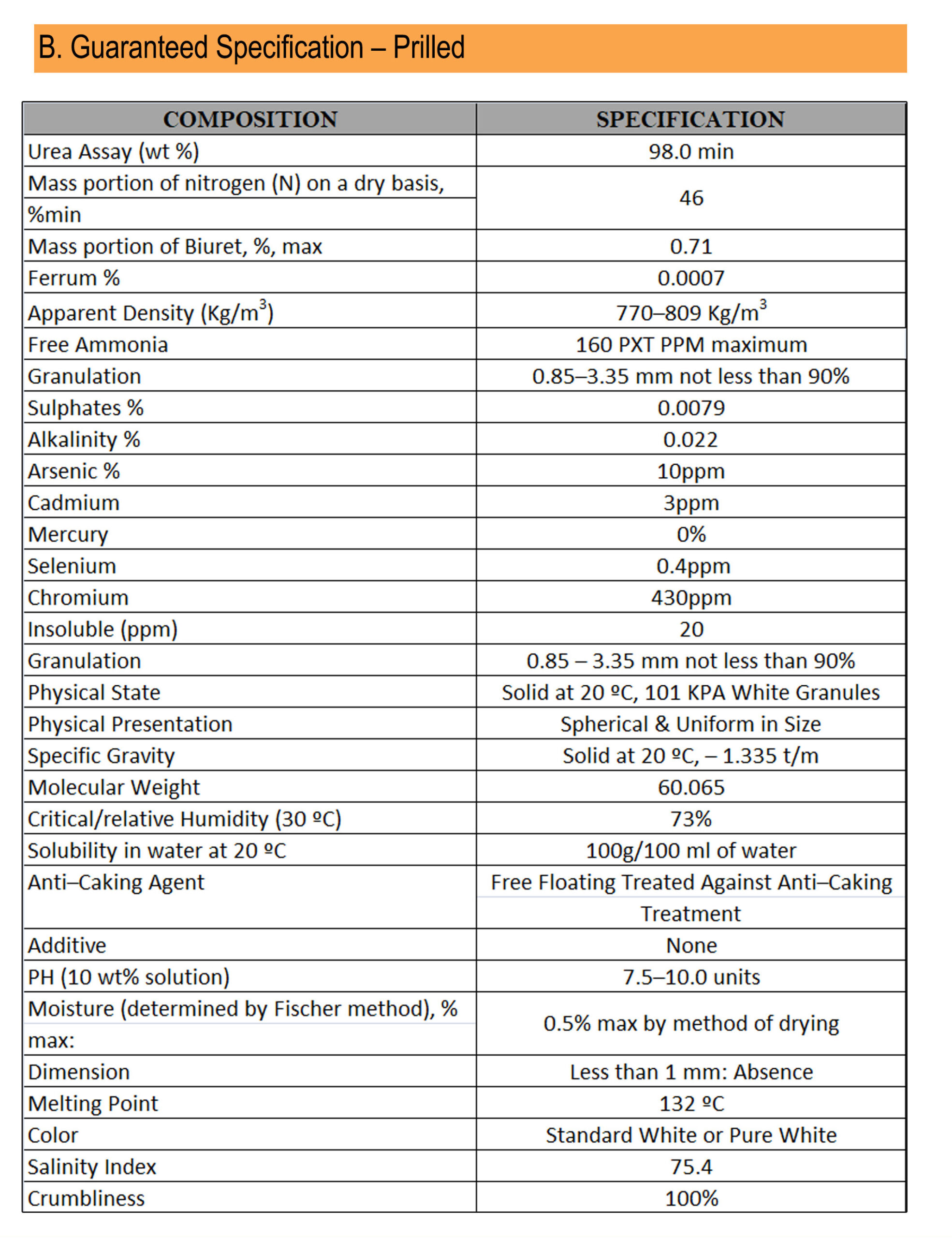
UREA
Urea is a nitrogen-containing compound that is widely known for its role as a key component in fertilizers. It is a colorless, odorless, crystalline substance with the chemical formula (NH₂)₂CO. Urea is highly soluble in water and is commonly used in both agriculture and industrial applications.
Here’s a more detailed overview of urea:
- Molecular formula: (NH₂)₂CO
- Appearance: White, odorless, crystalline solid
- Solubility: Highly soluble in water
- Melting point: Around 132°C (270°F)
- Boiling point: Decomposes at high temperatures rather than boiling
Urea is primarily synthesized through the Haber-Bosch process and the BASF process. The process involves combining ammonia (NH₃) and carbon dioxide (CO₂) under high pressure and temperature to produce urea.
Ammonia Synthesis: Ammonia (NH₃) is first produced using natural gas (methane) as a feedstock through the Haber-Bosch process, which combines nitrogen from the air and hydrogen (from natural gas) under high temperature and pressure.
Urea Synthesis: The ammonia is then reacted with carbon dioxide (CO₂) in a high-pressure reactor to produce urea. The reaction can be summarized as:
This produces ammonium carbamate, which is then dehydrated to form urea.
Uses of Urea:
Fertilizer (Agriculture):
Urea is primarily used as a nitrogen fertilizer in agriculture. It is one of the most widely used fertilizers globally due to its high nitrogen content.
Nitrogen Source: Nitrogen is a vital nutrient for plants, promoting growth, chlorophyll production, and overall health. Urea supplies nitrogen in a form that is easily absorbed by plants.
Forms: Urea can be applied directly to the soil or can be coated for slow release. It is also used in liquid fertilizers.


Chemical Industry:
Production of Resins: Urea is used in the production of urea-formaldehyde resins, which are used in adhesives, paints, and as a component in some plastics.
Feedstocks for Chemicals: It serves as a raw material for the production of other nitrogen-containing compounds like urea nitrate (used in explosives) and melamine (used in plastics and kitchenware).
Industrial Reactions: Urea is involved in the production of certain chemicals and is used in applications such as urea-based cleaning agents.
Pharmaceuticals:
Topical Treatments: Urea is used in some skin care products, especially in the treatment of dry skin, eczema, and psoriasis. It has moisturizing and keratolytic properties, meaning it helps in exfoliating the skin and promoting hydration.
Medications: Urea is also used in some medical applications, such as intravenous urea for the treatment of kidney failure or to reduce intracranial pressure.
Animal Feed:
Protein Supplement: Urea is used as a non-protein nitrogen source in livestock feed. It provides a form of nitrogen that ruminants (such as cows and sheep) can convert into protein through microbial activity in their stomachs.
Feed Additives: Urea is included in animal feeds to enhance protein levels and promote growth in cattle, poultry, and other livestock.
Diesel Exhaust Fluid (DEF):
Selective Catalytic Reduction (SCR): Urea is a key component of diesel exhaust fluid (DEF), which is used to reduce harmful emissions in diesel engines. When injected into the exhaust system, DEF reacts with nitrogen oxides (NOx) to convert them into harmless nitrogen and water.
Textiles:
Dye Fixing and Processing: In the textile industry, urea is used to aid in the dyeing process. It helps fix dyes onto fabrics and is involved in certain chemical treatments of textiles.
Food Industry:
Urea is used as a food additive in some processed foods (though in very small amounts) and can be found in ingredients like yeast nutrients for fermentation processes.

Urea in Industrial Processes:
Urea in Agriculture: As a fertilizer, urea is one of the most efficient nitrogen sources for plants. Its high nitrogen content and cost-effectiveness make it popular worldwide. However, managing its application is crucial to prevent environmental issues like soil acidification and nitrate leaching.
Industrial Production of Urea: Urea is produced in specialized chemical plants known as urea synthesis plants. These plants employ high-pressure and high-temperature reactions to convert ammonia and carbon dioxide into urea.